- Published:
- 18 January 2024
- Author:
- Dr Paula Bolton-Maggs, Dr Jennifer Davies, Dr Catherine Booth, Dr Josephine McCullagh, Simon Carter-Graham & Dr Shruthi Narayan
- Read time:
- 11 Mins
This article, by the UK’s Serious Hazards of Transfusion team, explores transfusion incidents in maternity and the themes that can be identified to improve patient safety.
Serious Hazards of Transfusion (SHOT) is the United Kingdom’s independent, professionally led haemovigilance scheme. SHOT has collected and analysed anonymised information on adverse events and reactions related to blood transfusion from all UK healthcare organisations involved in the transfusion of blood and blood components since 1996. SHOT’s remit is to receive and collate confidential reports of transfusion-related deaths and major complications. Where risks and problems are identified, SHOT produces recommendations to improve patient safety.
SHOT’s mission is to improve patient safety in blood transfusion by:
- improving standards of hospital transfusion practice
- educating users on transfusion hazards and their prevention
- aiding production of clinical guidelines
- informing policy within the UK Blood Services
- informing national policy on transfusion safety within the UK.
Further information about SHOT and all Annual SHOT Reports including educational resources can be accessed from the SHOT website. These are also available to access anytime via the SHOT UK app, which is free to download on any smart phone.
This article focuses on incidents reported to SHOT from maternity in key reporting categories. Recommendations for enhancing safety are also covered. Please note that this does not cover all incidents reported from maternity, but these categories have been included to highlight the key recurring themes to inform improvement initiatives that enhance patient safety.Wrong blood in tube (WBIT) are cases where blood is taken from the wrong patient and labelled with the intended patient’s details, or blood is taken from the intended patient but labelled with another patient’s details. These errors can result in ABO-incompatible transfusions, with potentially serious complications including death. Overall, for every case there are about 100 near-miss (NM) incidents.
Accurate patient identification is key. The patient should be asked at the time of venepuncture to state their first name, surname and date of birth. The unique patient identification number should be added to all requests and samples. SHOT’s WBIT NM data show that maternity departments and clinics are particularly high-risk areas. In 2019–2022, which included a total of 3,025 WBIT NM reports, 25–34% of the blood samples were taken by midwives.
2 errors account for more than 75% of cases: failure to identify the patient correctly at the time of venepuncture and labelling the sample away from the patient. In 2022, both errors were made together in nearly 20% of cases. Often understaffed and working in several areas including the community, midwives have a higher rate of failure to label samples at the patients’ side compared to other professional groups.
From 2019 to 2022, 152/190 (80%) of NM reports in children were in infants less than 28 days old. Infants on special care baby units may have similar dates of birth (or the same if twins) and may not yet have an allocated first name. There may also be a delay in allocating a unique patient identification number. There were 26 cases of confusion between twins.
Where maternal and baby samples are required to confirm ABO and D-groups, the baby sample is usually taken from the umbilical cord, often after the placenta and cord have been removed to the ward sluice area. The cord sample is then labelled away from both mother and her baby. In this 4-year period, there were 45 errors in labelling of cord and maternal blood samples.
Babies whose mothers are D-negative are at risk from haemolytic disease of the fetus and newborn if the baby is D-positive. The fetal D group can be identified from maternal blood samples early in pregnancy by analysing cell-free fetal DNA (cFFDNA). The blood group is then confirmed from a cord sample at birth. Comparison of these groups can detect errors:
- Example 1. Baby and maternal grouping samples showed that a baby was O D-negative, the same group recorded as the maternal blood. The cFFDNA test predicted the baby as D-positive. Further testing confirmed the baby group was O D-positive.
- Example 2. A baby was found to be B D-positive, with a known D-negative mother. The mother was prescribed anti-D immunoglobulin (Ig), but she knew that the child’s father was also D-negative. The baby was is bled again twice and both were grouped as A D-negative.
Errors in D grouping may result in inappropriate management. In 2020, 51 D-negative women grouped as D-positive. These women might have missed their anti-D Ig. There were 28 instances where a mother or baby was recorded as D-negative whose true group was D-positive. Of these errors, 3 related to mother and cord blood samples.
In conclusion, NM WBIT data demonstrate that maternity departments are high risk areas for mistakes in patient identification and sample labelling. The underlying causes may be high workload, inadequate staffing levels and insufficient identifiers for neonates and cords. Further information about NM events reported to SHOT and related resources can be found on the SHOT website.Anti-D Ig for D-negative mothers following the birth of a D-positive infant was introduced in the 1960s. It was instrumental in reducing risk of immunisation to the D-antigen resulting in haemolytic disease of the fetus and newborn, significantly reducing deaths of infants. Guidelines for the safe and appropriate administration of anti-D Ig post-sensitising events (PSE), including delivery, and routine antenatal anti-D Ig prophylaxis (RAADP) have now been in place for many years.1–4 It is essential that these guidelines are reflected in local policies and that systems are in place that support compliance in all healthcare settings.
SHOT data continue to demonstrate that errors in anti-D Ig and RAADP management occur in both clinical and laboratory settings. The management of anti-D Ig and RAADP is multifaceted; errors can occur at all stages of the process, including identification of the requirements, ordering, prescription, laboratory release, storage and administration. Omission or late administration of anti-D Ig or RAADP consistently account for the majority of cases reported, mainly relating to patient discharge prior to administration and to flawed decision making. Failure to identify the need for anti-D Ig following PSE is commonly noted in cases where the mother is seen by clinical teams in non-maternity or gynaecology settings.
The implementation of high-throughput, non-invasive cffDNA screening has improved practice by supporting targeted administration of this blood product to those who need it. This screening has reduced unnecessary exposure to mothers carrying D-negative fetuses and protected supplies of the product. Access to the screening program should now be an accepted standard in all maternity services.
IT systems have been increasingly implemented to support patient care in healthcare organisations. The power of these systems has been harnessed to support the safe and appropriate management of anti-D Ig. Laboratory computer systems should be developed and configured to support safe practice for the release of anti-D Ig when appropriate, based on patient D-type, cffDNA screening results and the absence of immune anti-D in maternal blood samples.
SHOT provides a variety of resources that can be used as training aids for healthcare staff. These are open access for patients and carers (such as the SHOT Bite and anti-D Aide-Memoire5,6). Mothers are often well informed on their pregnancies and should be included in discussions relating to anti-D Ig requirements, the importance of timings for administration and the risk of delays and omissions in administration. This topic has been discussed in detail previously in the College Bulletin.Although blood transfusion is generally very safe, alloimmunisation poses a risk of particular concern to women of childbearing potential. Red cell antibodies that develop following transfusion can potentially cause haemolytic disease of the fetus and newborn in future pregnancies; even where antibodies do not affect the baby, they can necessitate additional monitoring and cause significant anxiety. This makes it especially important to avoid transfusion in this patient group unless essential.
Anaemia during pregnancy is almost always due to iron deficiency (IDA), which is completely treatable provided it is detected and the appropriate iron supplements are provided. Women who are anaemic going into delivery are more likely to require transfusion in the event of significant blood loss.
Of the 15 avoidable red cell transfusions in pregnancy that were reported to SHOT between 2016 and 2022, 6 were related to mismanagement of iron deficiency in the antenatal period. There is undoubtedly under-reporting in this area. 3 women going into caesarean section were transfused due to significant IDA, which had not been adequately treated during the pregnancy. 2 stable patients received elective transfusions of multiple (2–3) units antenatally for IDA, where IV iron would have been more appropriate. 1 woman had no monitoring after her booking bloods and presented at 37 weeks with severe IDA, with haemoglobin at 42 g/L.
There can be a tendency to over-transfuse in the setting of post-partum anaemia merely to treat a low haemoglobin number. A young, healthy patient will compensate for a low haemoglobin and, with a normal bone marrow, will recover within weeks, provided they have sufficient raw materials (largely iron). A report mentioned that an asymptomatic 33-year-old who refused transfusion was given a unit due to a post-partum haemoglobin of 67 g/L. Unless there is haemodynamic instability, it is essential that the risks, benefits and alternatives to transfusion are discussed in detail before rushing to transfuse.
Further information about avoidable transfusions reported to SHOT and related resources can be found on our website.Over the last decade, the risk of obstetric haemorrhage has risen in developing countries (including the UK), owing mainly to increasing maternal age at the time of delivery, multiple births, obesity and increased obstetric interventions, such as caesarean section.7–9 Blood transfusion during major haemorrhage plays an integral role in the management of bleeding patients. Delays in the provision of blood components during emergency settings, such as major haemorrhage, can have detrimental effects on patients that can result in morbidity and mortality.
Delays in blood transfusion have been a cause of concern for some years now, as they are currently one of the leading causes of transfusion-related deaths.10 Over the past 5 years, the number of blood transfusion delays reported to SHOT has increased. A trend identified in these reports is delays associated with major haemorrhage protocols (Figure 1).
Figure 1. Delays in blood transfusion
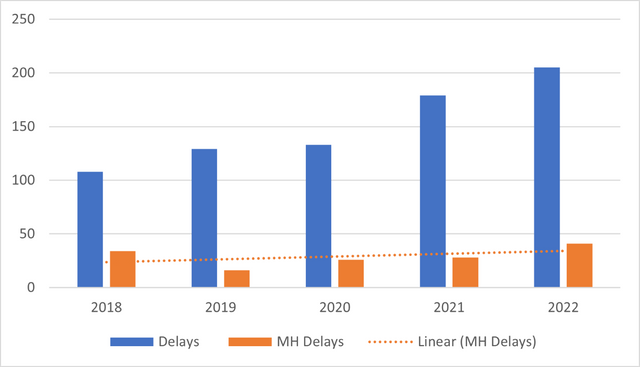
The most recent Annual SHOT Report10 for 2022 reported a total of 205 cases of delayed blood transfusions. 41 cases (20%) were errors associated with major haemorrhage and 6 (14.6%) of these were errors in the management of an obstetric haemorrhage. A common issue identified was failure in communication, which occurred in 50% of the cases.
Case 1
Blood components were requested for a woman with post-partum haemorrhage. There were 2 records on the system for the same patient. A lack of clear communication between the laboratory staff and the clinical team resulted in a delay in the issue of blood components. Furthermore, there was no communication within the team to collect emergency O neg units.
Case 2
The major haemorrhage protocol was activated for a woman with a placental abruption. Unclear instructions were provided to the porter transporting the units, resulting in a delay in the provision of blood components to the patient in theatre.
Case 3
Following the activation of the major haemorrhage protocol for a patient in maternity with a >2L blood loss, multiple phone calls to the transfusion laboratory were made by multiple different members of the clinical team. There were additional delays at several points in transporting the blood components to the clinical area.
In conclusion, delays in blood provision during a major haemorrhage are regularly identified through reporting. Poor communication during patient care is common and exacerbates delays in blood provision.The common themes in the preventable errors reported to SHOT demonstrate the real risk of harm to patients, especially in maternity. It is important to recognise that transfusion is a complex, multistep process that involves members of several professional groups, including nurses, midwives, doctors, laboratory scientists as well as blood donors and recipients.
Transfusion safety depends upon the coordinated linkage of all processes, from collection of the blood component from blood donors to transfusion in the recipient. There are a number of various steps in the transfusion pathway, from making the decision to transfuse to administrating blood components and monitoring/managing complications. Safe and effective communication, timely coordination and collaboration between all teams involved in patient care, both clinical and laboratory, are critical to ensuring transfusion safety.
Decision-making in transfusion practices can be improved with the use of checklists, by embedding electronic identification systems or by incorporating human factors and ergonomic principles. All healthcare organisations involved in transfusion are encouraged to continue implementing the key recommendations from the Annual SHOT Reports and to ensure that the measures have been effective. Learning from incidents should be optimised and shared widely. Additionally, patient education is crucial as it empowers individuals to actively participate in their care and fosters a stronger patient–provider relationship, promoting trust and collaborative decision-making.
References available on our website.
Return to January 2024 Bulletin homepage
Read next
Iron deficiency in women – the silent debilitator
18 January 2024