- Published:
- 15 July 2024
- Author:
- Dr Benjamin Swift
- Read time:
- 6 Mins
In this article, veterinary microbiologist Ben Swift explains a state-of-the-art approach to using bacteriophages to diagnose mycobacterial diseases. This pioneering diagnostic test has potential to benefit both human and veterinary diagnostics.
How can phages detect bacteria?
- The detection of mycobacteria using molecular methods, such as polymerase chain reaction (PCR), can be hampered by inefficient cell lysis.
- Our team has recently commercialised a bacteriophage technology, Actiphage, through PBD Biotech. This technology exploits phages’ ability to break open bacteria from the inside to efficiently lyse their host and release their DNA, without the need for chemical or mechanical lysis methods.
- We can then simply carry out a PCR assay to detect the DNA released; crucially, as phages only replicate inside a live host, only DNA from the viable host will be detected, which is akin to culture (Figure 1).1
Members of the Mycobacterium genus are responsible for some of the world’s most challenging diseases in humans and animals. M. tuberculosis – the causative agent of tuberculosis (TB) – is the second most deadly infectious disease in humans worldwide. Currently second to COVID-19, TB is expected to regain the top spot in the near future.
M. bovis – a close relative of M. tuberculosis - is the causative agent of bovine tuberculosis (bTB). This is primarily a disease of cattle but can infect a range of species, including humans. It is estimated that there were 143,000 cases and 12,300 deaths in humans due to M. bovis in 2018.2,3 bTB is included in the list of mandatory notifiable diseases of the World Organisation for Animal Health (WOAH).4 In addition to threatening public health, bTB is also a significant economic concern. bTB is estimated to cost $3 billion worldwide annually, owing to reduced productivity, culling and movement and trade restrictions. In the UK alone, 44,656 cattle were culled in 2018.5
Another significant disease in animals caused by mycobacteria is Johne’s disease – caused by M. avium subsp. paratuberculosis (MAP). Johne’s disease is considered endemic in many countries worldwide and has been linked to Crohn’s disease in humans.6
Mycobacteria are characterised by an extremely slow growth rate – often taking weeks or months to grow on artificial media. Their thick, waxy cell walls aid in their ability to avoid their host’s immune response, make them impenetrable to a range of antibiotics7 and, crucially for molecular diagnostics, make them difficult to break open.8
Although classical laboratory culture of mycobacteria is generally considered the gold-standard method of detection, in practice it is not very practical because of their slow growth rate, staff health and safety considerations and potential for cross-contamination. It also has high failure rates, owing to potential contaminants. Furthermore, cultures of M. tuberculosis and M. bovis must take place in Containment Level 3 facilities, adding to cost and practicality issues.
Many current diagnostic tests for mycobacteria used in veterinary medicine are immunologically based – such as enzyme-linked immunoassays (ELISAs). However, there can be issues with cross-reactions occurring if the animals have been exposed to other pathogenic or environmental mycobacteria, which may result in false-positive results. Another issue is that, depending on the stage of infection, sensitivity of these detection methods can be very low.9,10
Timely and accurate detection of mycobacterial infections is crucial for effective disease management, yet traditional diagnostic methods often fall short in terms of sensitivity, specificity and speed. Phage technology offers a promising solution to these limitations.
Actiphage detection offers several key advantages over traditional diagnostic methods for mycobacterial infections.
Rapid detection
Actiphage delivers results within hours, enabling timely intervention and reducing the risk of disease progression and transmission.
High sensitivity
Actiphage demonstrates exceptional sensitivity and is capable of detecting mycobacterial loads as low as a single viable cell. This sensitivity is particularly valuable in early-stage infections or cases where bacterial burdens are low, which enhances diagnostic accuracy and reduces the likelihood of false negatives.
Species specificity
The method exhibits remarkable specificity for mycobacteria, distinguishing them from other bacterial species present in complex clinical samples. Coupled with a specific end-point nucleic acid amplification method, this method can be extremely specific.
Versatility
This method is adaptable to various sample types, including blood, tissue and environmental samples, making it suitable for diagnosing mycobacterial infections across diverse clinical and veterinary settings. Moreover, its compatibility with molecular detection methods facilitates integration into existing laboratory workflows.
Bacteriophages have been explored as an alternative or complementary treatment for antibiotic-resistant infections. During my research, however, we have explored the use of bacteriophages as a promising technique to reduce the time for, and improve the accuracy of, diagnosis, even in the early stages of disease.
Phages are viruses that infect bacteria. They will only successfully replicate within a live-viable host. Phages can be extremely specific and identify their host cells by binding to specific receptors on their surface. Once a phage has successfully bound to its host, it injects its nucleic acid into the bacterial cell and hijacks the cellular machinery to rapidly reproduce. Once the phages have replicated within their host, they encode enzymes to break down their host’s cell wall from the inside to allow the release of progeny phages.
Phages have been used for many years to rapidly detect different bacterial pathogens, including members of the Mycobacterium genus.11 Several different phage-based detection assays have been described. One assay uses genetically engineered reporter-phages that produce a fluorescent or bioluminescent signal when they infect their host cells; another binds phages to host cells that can be detected using physical methods, such as plasmon resonance.9
However, more recently, the detection of mycobacteria using bacteriophage amplification technology has been developed, where the natural life cycle of the phage is exploited to detect its bacterial host. One such example is our recently developed Actiphage.
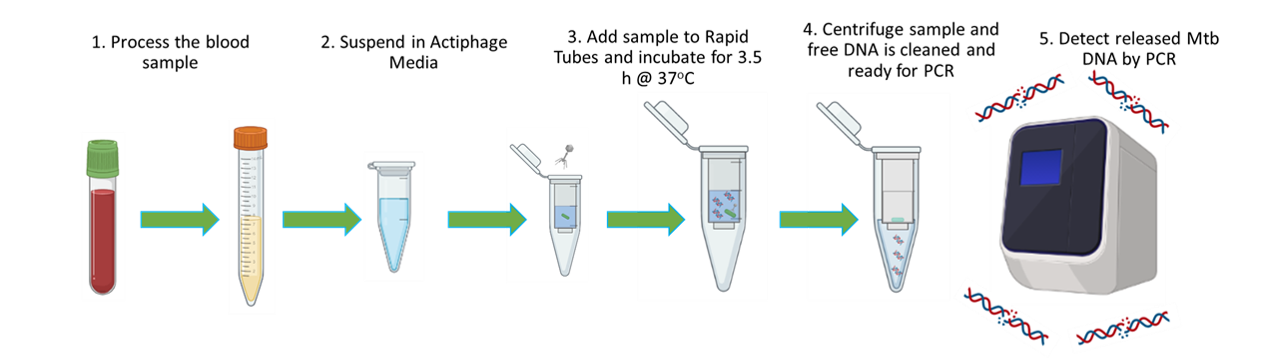
Figure 1. Actiphage schematic. 1: Blood preparation; 2: lysis of peripheral blood mononuclear cells; 3: addition of phage. For the Actiphage assay, the sample is incubated for 3.5 h at 37 °C to allow the phage to complete its replication cycle and lyse the mycobacterial cells. 4: Any intact bacterial cells are separated from free genomic DNA released after phage by filtration and then the DNA is further cleaned and concentrated using a commercial kit. 5: Finally, signature sequences from the mycobacterial genomic DNA are detected by PCR.
This method presents a game-changing tool for the diagnosis and control of mycobacterial infections in livestock and wildlife. By accurately identifying infected animals, including those in the early stages of infection or with low bacterial burdens, bacteriophage amplification technology can support targeted disease management strategies, such as herd segregation, test-and-cull programs and vaccination campaigns. Moreover, its application in wildlife surveillance programmes facilitates the detection and control of bTB in reservoir hosts, thereby reducing the risk of spillover into domestic livestock and human populations.
Our research on Actiphage was recognised by Applied Microbiology International, where I received the Basil Jarvis Horizon Award. This award recognises work that addresses part of the 2nd United Nations Sustainable Development Goal to tackle hunger worldwide.
Early studies in both active TB patients and contacts have demonstrated Actiphage’s promise as an extra tool to tackle TB.12,13
Not only might it offer rapid and sensitive detection of M. tuberculosis in clinical samples but it can also detect drug-resistant strains of M. tuberculosis, which could offer support for treatment optimisation.
Beyond TB, bacteriophage amplification technology may find applications in diagnosing other mycobacterial infections that affect humans, including non-tuberculous mycobacterial (NTM) diseases, such as M. avium complex infections. NTM infections pose diagnostic challenges owing to their diverse clinical presentations and slow growth in culture, highlighting the need for rapid and sensitive diagnostic methods such as this.
Ongoing research efforts aim to further optimise and validate Actiphage for broader clinical and veterinary use. This includes refining assay protocols, expanding its applicability to additional mycobacterial species and exploring its integration into point-of-care testing platforms for decentralised diagnostics. Additionally, continued investment in capacity-building and technology transfer initiatives is essential to ensure equitable access to these diagnostics in resource-limited settings, where mycobacterial infections pose significant public health challenges.
In conclusion, bacteriophage amplification technology represents an exciting advance in the field of mycobacterial diagnostics. It offers rapid, sensitive and species-specific detection of difficult pathogens in both human and animal populations. By accelerating diagnosis, guiding treatment decisions, and informing disease control measures, it has the potential to mitigate the burden of mycobacterial infections.
References available on our website.
Return to the July 2024 Bulletin
Read next
An update on mRNA cancer vaccines
15 July 2024