Background and challenge
Statins have been the main evidenced-based treatment of patients with hypercholesterolaemia since the mid-1990s. Statins have a proven track record of reducing the risk of developing cardiovascular disease (CVD), the recurrence of CVD events in patients with established ischemic heart disease (IHD) and also those with genetically inherited familial hypercholesterolaemia (FH) with or without established CVD.1 Although statins are effective and well tolerated in most patients, a significant number are either unable to tolerate statins or have not reached therapeutic total cholesterol (TC) and low-density lipoprotein-cholesterol (LDL-C) targets and hence remain at high risk of a cardiovascular event.
I established and have been running a lipid clinic service in the Cwm Taf University Health Board since 1995 and hence have a significant number of patients in both of these groups, who have posed a long-term therapeutic challenge and whom I have been unable to treat optimally.
In 2016, however, NICE approved a new class of injectable lipid-lowering drugs called proprotein convertase subtilisin/kexin type 9 inhibitors (PCSK9-I) for these specific patient groups.2,3 PCSK9-I are monoclonal antibodies and have a different mode of action to statins. Statins inhibit hepatic cholesterol production, stimulating LDL-receptor (LDL-r) synthesis, whereas PCSK9-I inhibit low density LDL-r metabolism to decrease LDL-C (figure 1). Two PCSK9-I drugs have been licensed for treatment in the UK: alirocumab and evolocumab.
Figure 1: Mode of action of statins and PCSK9 inhibitors in the liver
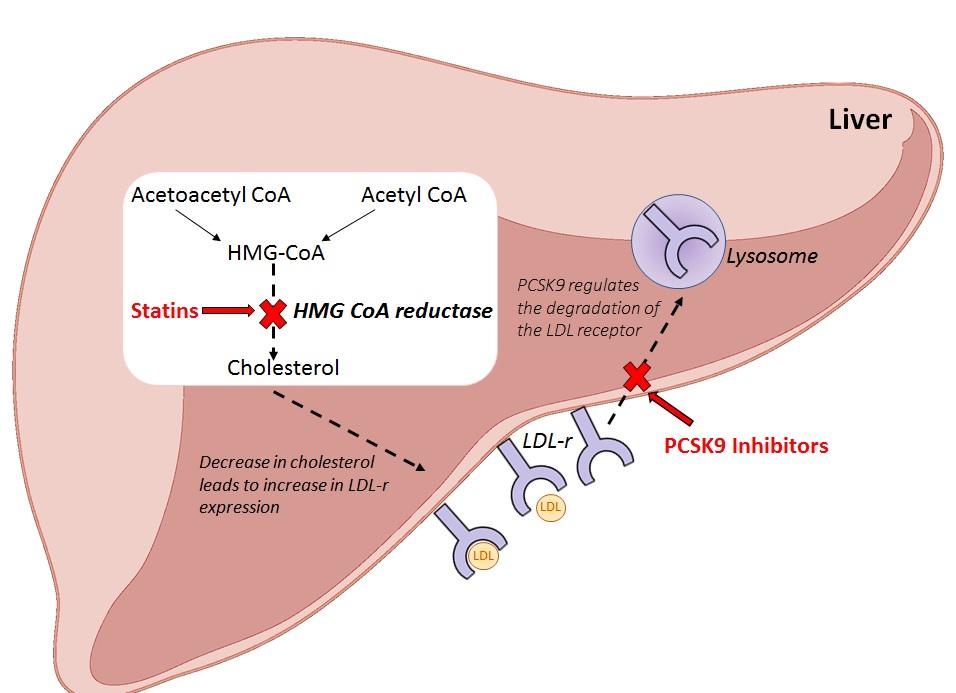
NICE has approved the use of PCSK9-I treatment for those patients with CVD or primary heterozygous FH with or without CVD who have tried at least two statins but are intolerant or have failed to meet LDL-C targets.2,3
As with many new treatments, however, there is a significant cost difference between PCSK9-I and statin treatments. The cost of statins varies between £10 and £350 per patient per year, which compares to PCSK9-I treatment at approximately £4,000 per patient per year (tables 1 and 2). The challenge, therefore, was to develop and implement a viable and sustainable service to benefit eligible patients within the catchment area of the Health Board.
Table 1: Prices of statins taken from the electronic drug tariff (as on 11.04.18)
|
Statin |
Cost for 28 days |
Cost for one year |
|
Atorvastatin 40 mg |
£1.19 |
£14.28 |
|
Atorvastatin 80 mg |
£1.89 |
£22.68 |
|
Simvastatin 40 mg |
£0.86 |
£10.32 |
|
Simvastatin 80 mg |
£1.74 |
£20.88 |
|
Pravastatin 40 mg |
£1.26 |
£15.12 |
|
Rosuvastatin 40 mg |
£26.02 |
£312.24 |
|
Rosuvastatin 80 mg |
£29.69 |
£356.28 |
Table 2: Prices of PCSK9 inhibitors taken from online BNF (as on 11.04.18)
|
PCSK9 inhibitor |
Cost for 28 days |
Cost for one year |
|
Evolocumab 140 mg/ml pre-filled syringe (based on 140 mg fortnightly) |
£1.19 |
£14.28 |
|
Alirocumab 75 mg pre-filled pen) based on 75 mg fortnightly) |
£1.89 |
£22.68 |
|
Alirocumab 150 mg pre-filled pen (based on 150 mg fortnightly) |
£0.86 |
£10.32 |
Cwm Taf PCSK9-I service development
Working with a senior clinical pharmacist and three registered nurses from our Cardiac Day Case Unit (CDCU), a multidisciplinary team was established. With the support of the Pathology and Pharmacy Directorates, based on the established clinical need and an estimated number of patients eligible for treatment, a costed and financially viable service development proposal was submitted and approved by the Health Board’s Medicines
Management and Efficiency Committee (MMEC). Following Health Board approval, a patient criteria form consistent with NICE guidelines2,3 was developed, along with protocols and procedures for the ordering, storing, handling and prescribing of the drugs. At the same time, the three nurses in our team undertook training to learn about PCSK9-Is, how they work, the rationale for treatment and the injection procedures.
In collaboration with the Cardiology department, a regular time slot to run the clinic in the CDCU was established. One of the key aims of the service development was to provide an appropriate environment to start patients on this new type of treatment with sufficient time to discuss the rationale for the treatment, and to teach the patient how to safely administer the injection and to appropriately store the medication. The logistics and patient administration of the service was set up with the medical secretarial staff in the Clinical Biochemistry department.
This preparatory work commenced in 2015 and was completed to coincide with NICE approval in April 2016.
Service delivery
The service commenced in August 2016. All patients started on the PCSK9-I treatment to date have been selected from the established lipid clinic. They have all been tried on numerous statins, including in combination with ezetimibe and other lipid lowering drugs, using varying treatment regimens and have either been intolerant of treatment or failed to reach therapeutic targets on the maximum tolerated dose and hence meet the NICE guidance criteria for PCSK9-I treatment. In the clinic the PCSK9-I treatment option is discussed and if the patient is happy to proceed, arrangements are made to commence.
Patients are invited to attend the CDCU four times at two-weekly intervals when initiating treatment to ensure they are confident with the process and self-injection. Patients are encouraged to contact the team if any problems arise or to raise any queries about ongoing treatment and potential adverse effects. Following the initiation period, patients are given a three-month prescription provided by the clinical team prior to review in the lipid clinic.
Consistent with the directive of the MMEC, as the sole designated PCSK9-I prescriber, I am responsible for the ongoing prescribing, working closely with the other team members from the CDCU, Pharmacy and Clinical Biochemistry Department to ensure the smooth provision of repeat prescriptions. Each prescription is vetted by the senior clinical pharmacist to ensure appropriate prescribing and dosing regimens and tight fiscal control. An audit trail is kept on the procedures and patients are monitored clinically and biochemically on a monthly basis prior to returning to the lipid clinic for a subsequent six-monthly follow-up. Patients are also able to discuss any ongoing concerns between clinic visits in order to document any problems.
Service progress and outcomes
At the time of writing, 40 patients have been commenced on one of the two PCSK9-I injections. These have all been from the lipid clinic. Of the 40 patients, 20 are on evolocumab 140 mg, and 20 on alirocumab 75 mg or 150 mg fortnightly respectively.
The impact on the TC and LDL-C has been significant, with the LDL-C reduction ranging from 40% to 60%. The majority of patients have tolerated the treatment and adapted to self-injection without problems. Two patients have stopped treatment; the first owing to muscle pains, as experienced with previous lipid-modifying regimen, and one with pain around the injection site and nausea.
At the outset the estimated number of patients requiring PCSK9-I treatment was 30–40 per year and it is anticipated that 50–60 patients will be receiving PCSK9-I treatment by August 2018. It is anticipated that most of the patients requiring PCSK9-I treatment will continue to come from the Lipid Clinic, most of whom are general practitioner referrals owing to patient intolerance or failure to meet therapeutic targets, but also an increasing number of referrals from cardiologists and diabetologists.
The Health Board has determined that the service remains under the direction and management of the established multidisciplinary team to ensure continued compliance with patient selection and fiscal control.
Reflection and conclusion
The primary objective has been achieved by excellent multidisciplinary team-working and the team acknowledge the support of Cwm Taf University Health Board. Patient benefits have been realised and the programme provides a framework for further service development with ongoing clinical audit and financial monitoring.
References for this article are available on our references pages.

