- Homepage
- Profession
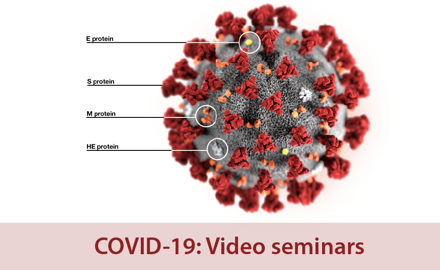
- Coronavirus: COVID-19 resources hub
Coronavirus: COVID-19 resources hub
This page contains information available on COVID-19 relevant to the practice of pathology. Many of the resources were developed during the earlier stages of the pandemic before the roll out of the vaccine programme and when little was known about the impact of infection from SARS-CoV-2. As the COVID pandemic moves into a different phase and with restrictions and infection rates varying across the UK, the guidance should be amended and applied as necessary in response to local rates and risk from COVID-19 infection.. The clinical risk of departing from any guidance should be assessed and documented.
We have also setup a page for trainees, which contains College guidance and advice about COVID-19 relevant to trainees.
If you notice any out-of-date information, or are aware of guidance and advice that would be relevant to the membership that we do not currently list here, please contact us at [email protected].
Public Health England and the Department of Health and Social Care provide the latest information on COVID-19 for health professionals, including guidance on the assessment and management of suspected UK cases.
Documents and resources are available by clicking on the drop-down arrow under each heading.

Clinical guidance and advice
General guidance
Resources: General guidance
-
RCPath briefing on LAMP assays
November 2020
-
COVID-19: safe handling and processing for samples in laboratories
This interim guidance from PHE is specific to clinical diagnostic laboratory practice in England and may differ from guidance produced by agencies in other countries, including recommendations about containment levels and control measures.
Public Health England
-
RCPath suggestions for COVID-19 antibody assay clinical comments
Consistent messages about the interpretation of positive SARS-CoV-2 antibody are important. This is especially so with the lack of current data to associate any such positive result with clear evidence of protection against reinfection.
May 2020
-
RCPath guidance on the de-isolation and discharge of COVID-19 patients
This guidance is based on documentation produced by East Kent Hospitals University NHS Foundation Trust. It follows Public Health England guidance. Version 2 – Updated 13 May 2020.
May 2020
-
RCPath Algorithm for symptomatic staff, household testing and further actions
This document contains an algorithm, adapted from PHE guidance, for testing symptomatic staff and further actions dependent on results.
April 2020
-
COVID-19: management of exposed healthcare workers and patients in hospital settings
Guidance on the management of hospital staff and patients who have been exposed to COVID-19.
Public Health England April 2020
-
Minimum operating standards – novel coronavirus (COVID-19) patient pathway
This document sets out the minimum operating standards for each element of the patient pathway, from identification of a possible COVID-19 case, through coordination of required steps to discharge.
NHS England
-
Clinical guidance on COVID-19 SNOMED codes
In response to the COVID-19 pandemic, the Professional Record Standards Body (PRSB) has published guidance for health and care professionals in collaboration with NHS Digital.
Professional Record Standards Body
-
RCPath guidance for remote digital pathology
This guidance document outlines the recommendations of the Royal College of Pathologists’ Digital Pathology Committee regarding temporary remote reporting of digital slides in times of clinical and service necessity.
RCPath Digital Pathology Committee March 2020
-
Guidance to support recording of COVID-19 data in care records
This guidance covers terminology for recording COVID-19 information.
The Professional Record Standards Body (PRSB) and NHS Digital
-
How tests and testing kits for coronavirus (COVID-19) work
Information about COVID-19 tests and testing kits, including how they work, the different types of tests and the specifications manufacturers need to follow.
Medicines and Healthcare Products Regulatory Agency May 2020
#TestingMethods2020
The College, in partnership with the Department of Health and Social Care, the UK Bioindustry Association, British In Vitro Diagnostics Association, has setup a sourcing platform to seek new and novel solutions to help deliver 100,000 coronavirus tests a day by the end of April.
You can register to contribute in seconds, either with your email address or by signing in with your social media account (Twitter, Facebook, Google or LinkedIn). If you would prefer to put your idea forward confidentially to the closed part of this website, you can do that too.
Please do tell other people who might be able to contribute. You can tweet about the platform using the hashtag #TestingMethods2020.
Resources: #TestingMethods2020
-
The Testing Methods Sourcing Platform
This platform is a partnership between the Department of Health and Social Care, the UK Bioindustry Association, British In Vitro Diagnostics Association and the Royal College of Pathologists. It is seeking new and novel solutions to help deliver 100,000 coronavirus tests a day by the end of April.
-
COVID-19 Testing Methods Bulletin No 1
Prof Dame Sue Hill, Prof Jo Martin April 2020
-
COVID-19 Testing Methods Bulletin No 2
Prof Dame Sue Hill, Prof Jo Martin April 2020
-
COVID-19 Testing Methods Bulletin No 3
Prof Dame Sue Hill, Prof Jo Martin May 2020
-
COVID-19 Testing Methods Bulletin No 4
Prof Dame Sue Hill, Prof Jo Martin May 2020
-
COVID-19 Testing Methods Bulletin No 5 - 15 May
Professor Dame Sue Hill, Professor Jo Martin May 2020
-
COVID-19 Testing Methods Bulletin No 6
Prof Dame Sue Hill, Prof Jo Martin June 2020
-
COVID-19 Testing Methods Bulletin No 7 – 15 June
Professor Dame Sue Hill, Professor Jo Martin June 2020
-
COVID-19 Testing Methods Bulletin No 8 – 29 June
Professor Dame Sue Hill, Professor Jo Martin June 2020
-
COVID-19 Testing Methods Bulletin 9 – 24 July
Professor Dame Sue Hill, Professor Jo Martin July 2020
Infection prevention and control
This webinar shared the latest validation and evaluation data of LAMP and LFDs by the experts who performed the studies and analysis.
Resources: Infection prevention and control
-
Letter: NHS personal protection equipment, 28 March 2020
This letter from the National Medical Director of NHS England and NHS Improvement, Medical Director and Director of Health Protection of Public Health England and the Chair of the Academy of Medical Royal College about the supply and safety of, and guidance related to, NHS personal protective equipment.
NHSE and NHSI, PHE and AoMRC March 2020
-
COVID-19: infection prevention and control (IPC)
Guidance on infection prevention and control for COVID-19. Sustained community transmission is occurring across the UK.
Public Health England August 2020
-
COVID-19: infection prevention and control
Guidance from Public Health England on infection prevention and control for COVID-19, including the use of personal protective equipment.
Public Health England
-
Use of vacuum tubes in pathology – change in guidance
The previous version of this guidance from HSE regarding the use of pneumatic tubes for the transport of COVID-19 samples has been amended. The prohibition of their use for COVID-19 samples has been removed in this version – the latest edition as of 23 March 2020. All pathology services with pneumatic tubes should now be using this guidance.
Health & Safety Executive
-
RCPath guidance on the use of POCT equipment in COVID-19-positive patients
This guidance is based on documentation produced by the Royal Free London NHS Foundation Trust for the use of point of care tests for confirmed or suspected COVID-19 patients. It should be adapted for local use depending on the clinical setting and range of POCT in use.
March 2020
Histopathology specimens, sections and slides
Resources: Histopathology specimens, sections and slides
-
RCPath advice on histopathology frozen sections and cytology FNA during infectious disease outbreaks
This document provides clarification on the procedures related to frozen sections and fine needle aspiration during the COVID-19 outbreak, to reduce the risk of transmission of infection between healthcare staff.
March 2020
-
RCPath advice on the opening of unfixed histopathological specimens during infectious disease outbreaks
This advice is provided to help reduce the risk of transmission of infection between healthcare staff and patients via histopathological specimens during the COVID-19 outbreak.
March 2020
-
RCPath advice on the management of histology slides, request forms and microscopes during infectious disease outbreaks
This document clarifies RCPath advice on handling slides, request forms and microscopes during the COVID-19 outbreak.
March 2020
Care of the deceased
Resources: Care of the deceased
-
Chief Coroner's Guidance No: 37: COVID-19 deaths and possible exposure in the workplace
This document provides guidance relating to COVID-19 deaths where exposure may have occurred as a result of the individual's employment.
HHJ Mark Lucraft QC, Chief Coroner April 2020
-
Guidance on the Coronavirus Act 2020: implications for death certification, registration and cremation forms
The Coronavirus Act 2020 made changes to legislation about how deaths can be certified and registered during the pandemic. This document provides links to guidance on infection control and PPE, death certification and cremation forms, working with the Coroner, and background to the Act.
April 2020
-
Coronavirus (COVID-19): verification of death in times of emergency
This document clarifies existing practice for verifying deaths outside of hospitals and providing a framework for safe verification of death during the coronavirus emergency.
Department of Health and Social Care
-
G199 Cause of death list
The document offers guidance for those completing death certificates and those registering deaths. list details some of the conditions that may be included on the Medical Certificate of Cause of Death.
July 2020
-
RCPath Briefing on COVID-19 – autopsy practice – February 2020
This briefing is for mortuary staff who are potentially exposed to material including body fluids from the deceased in the mortuary.
February 2020
-
Transmission-based precautions: Guidance for care of deceased during COVID-19 pandemic
This guidance was produced by the Royal College of Pathologists and the Association for Anatomical Pathology Technology in conjunction with Public Health England.
RCPath and AAPT March 2020
-
Guidance for care of the deceased with suspected or confirmed coronavirus (COVID-19)
This advice is primarily designed to assist people who are required to manage the bodies of deceased persons infected with coronavirus (COVID-19).
Public Health England April 2020
-
Guidance on the Post Mortem sector licensing standards
This updated guidance includes additional information and examples of how licensing standards can be met. It is based on findings from inspections, incidents and enquiries, and was developed with HTA's Histopathology Working Group.
Human Tissue Authority April 2020
-
HTA licensing of emergency mortuary facilities
This guidance sets out key information about HTA licensing of emergency mortuaries, for teams involved in emergency planning and those working in emergency mortuaries.
Human Tissue Authority April 2020
Help us build a COVID-19 knowledge database: submit your post-mortem reports
We have developed a post-mortem case submission portal as a means to build a database of information to help inform the treatment of COVID-19 patients and research about the disease.
You can submit preliminary and authorised reports, as well as accompanying images, using the portal. Our team of pathologists will review all submissions, extract the salient information and add data to a findings document.
Specialty specific guidance
Placental changes of chronic histiocytic intervillositis & COVID-19
Recent cases have been reported which suggest a possible association between maternal COVID-19 infection and placental changes of chronic histiocytic intervillositis, sometimes with overlap with massive perivillous fibrin deposition. If placental examination reveals such pathology immunohistochemical staining to detect SARS-CoV-2 may be indicated.
MBRRACE-UK and our own perinatal leads within the College together with other stakeholders are monitoring the situation.
Resources: Specialty specific guidance
-
Haematology COVID-19 vaccination statement
January 2021
-
RCPath Recommendations for verification validation of sample-sets assays SARS-CoV-2
This guidance is produced to assist NHS and research laboratories to evaluate immunoassays for clinical use and help to meet ISO accreditation standards for clinical use. Version 2 – updated 26 May 2020.
May 2020
-
RCPath Immunology SAC statement on COVID-19 SARS CoV2 antibody evaluation
Statement from the Immunology SAC on antibody evaluation to ensure that assay evaluations are robust and fit for purpose.
April 2020
-
RCPath guidance on use and interpretation of clinical biochemistry tests in patients with COVID-19 infection
This document outlines the biochemical tests that have a role in the assessment and monitoring of patients with COVID-19 infection. It is intended to assist clinicians who are less familiar with these tests to understand the role and limitations of these markers in the management of patients with COVID-19.
April 2020
-
COVID-19 and neurological disease – implications for neuropathologists
This resource points to a selection of publications (including opinion pieces) related to the role of the CNS in coronavirus infections. It is not intended to be comprehensive or to give an opinion on the levels of evidence presented in the articles, but to serve as an ‘entry point’ to the topic of neuropathology in relation to COVID-19 and to stimulate discussion.
April 2020
-
Webinar – COVID-19: An unexpected pandemic challenge to unprepared health care systems
This webinar was recorded on 10 April and discussed numerous aspects of the COVID-19 pandemic that has gripped the globe over the past several months, including testing, preparedness and infection prevention, case management and treatment, and hospital and patient management.
ESCMID
-
COVID-19 rapid guideline: delivery of systemic anticancer treatments
The purpose of this guideline is to maximise the safety of patients with cancer and make the best use of NHS resources, while protecting staff from infection. It will also enable services to match the capacity for cancer treatment to patient needs if services become limited because of the COVID-19 pandemic.
National Institute for Health and Care Excellence
-
Recommendations for the management of patients with acute myeloid leukaemia (AML) during the COVID-19 outbreak
This guidance identifies treatment strategies in adults with newly diagnosed AML which reduce NHS pressure whilst preserving patient outcomes. Mindful of the pace of change these recommendations will undergo regular review and should be considered valid until 5 April 2020.
NCRI AML Working Party March 2020
-
UK best practice guidelines for reintroduction of routine fertility treatments during the COVID-19 pandemic
The BFS and ARCS recommend that this guideline should be used in conjunction with prevailing Government, health service and HFEA advice and regulations, to ensure safe and sustainable service delivery during the ongoing pandemic.
The Association of Reproductive and Clinical Scientists (ARCS) and British Fertility Society (BFS) June 2020
-
COVID-19 and fertility treatment: guidance for professionals
This guidance is for all fertility professionals and has been put together in conjuction oth the British Fertility Society (BFS), the Association of Reproductive and Clinical Scientists (ARCS) and the Royal College of Obstetricians and Gynaecologists.
Human Fertilisation & Embryology Authority
-
The dynamics of humoral immune responses following SARS-CoV-2 infection and the potential for reinfection
A new review discusses the findings from over 40 studies on coronavirus immunity and what they could mean for the COVID-19 pandemic.
Microbiology Society May 2020
Operational guidance and advice
Resources: Operational guidance and advice
-
SARS-CoV-2 routes of transmission and recommendations for preventing acquisition
Joint British Infection Association (BIA), Healthcare Infection Society (HIS), Infection Prevention Society (IPS) and Royal College of Pathologists (RCPath) guidance. April 2021
-
Working safely during coronavirus (COVID-19)
Guidance for people who work in or run indoor labs and research facilities and similar environments.
Department for Business, Energy & Industrial Strategy May 2020
-
RCPath COVID-19 and potential redeployment of pathologists
This document offers guidance and advice to pathologists facing potential redeployment during the COVID-19 outbreak.
March 2020
-
Redeploying your secondary care medical workforce safely
This guidance supports trusts to safely redeploy their secondary care medical workforce during the COVID-19 outbreak. It sets out the level of supervision staff may need if they are redeployed, to ensure they still work within their competency.
NHS England and NHS Improvement
-
Clinical academics and COVID-19: information and guidance
This information and guidance has been provided on behalf of the major research funders and wider research community across the four nations.
The Royal College of Physicians
-
COVID-19 business continuity updates from NHSBT
This page contains information and updates to help clinical and laboratory staff manage the impact of COVID-19, including current blood stock levels.
NHS Blood and Transplant
-
RCPath advice on MDT meetings during infectious disease outbreaks
This document provides advice for pathologists working as part of multidisciplinary teams during infectious disease outbreaks.
March 2020
-
Letter: COVID-19 testing to support retention of NHS staff, 29 March 2020
Amanda Pritchard, Stephen Powis, Sarah-Jane Marsh
Devolved nations information
Resources: Devolved nations information
-
Updated guidance on patients and family after death during the COVID-19 pandemic
The Academy of Medical Royal Colleges and Faculties in Scotland May 2020
-
Welsh Government information about COVID-19 in Wales
Welsh Government
-
Scottish Government information about COVID-19 in Scotland
Scottish Government
-
Public Health Agency information about COVID-19 in Northern Ireland
Public Health Agency
International resources
International resources
-
Escalating infection control response to the rapidly evolving epidemiology of the Coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong
This manuscript, accepted by the journal Infection Control and Hospital Epidemiology, describes infection prevention measures taken to prevent spread of COVID-19 infection in hospitals in Hong Kong.
Cheng VCC, et al.
Letters and statements
Resources: Letters and statements
-
Letter: Death Investigations Committee regarding about statistics on deaths during the COVID-19 pandemic, 12 May 2020
This letter raises concerns about statistics on deaths ahead of the hearing from the National Statistician, Professor Sir Ian Diamond, and Ed Humpherson, Director General for Regulation at the UK Statistics Authority.
RCPath Death Investigations Committee May 2020
-
Letter: Lord Bethell to NHS and PHE laboratory community, 6 May 2020
This letter recognises what is described as the 'phenomenal contribution' of NHS and PHE laboratories during the COVID-19 pandemic.
Lord Bethell May 2020
-
Letter: Joint statement regarding workforce response to coronavirus, 30 March 2020
This letter is a joint statement regarding the workforce response to coronavirus from the Chief Scientific Officer of NHS England and NHS Improvement, the Chief Scientific Advisor (Health) of the Welsh Government, the Chief Scientific Advisor of the Department of Health Northern Ireland and the Healthcare Science Officer, Scottish Government.
Sue Hill, Rob Orford, Ian Young, Karen Stewart March 2020
-
Letter: COVID-19 testing to support retention of NHS staff, 29 March 2020
Amanda Pritchard, Stephen Powis, Sarah-Jane Marsh
-
Letter: NHS personal protection equipment, 28 March 2020
This letter from the National Medical Director of NHS England and NHS Improvement, Medical Director and Director of Health Protection of Public Health England and the Chair of the Academy of Medical Royal College about the supply and safety of, and guidance related to, NHS personal protective equipment.
NHSE and NHSI, PHE and AoMRC March 2020
-
Letter: Important and urgent – next steps on NHS response, 17 March 2020
This letter sets out important actions that NHS England and NHS Improvement are asking every part of the NHS to put in place, to redirect staff and resources, building on multiple actions already in train.
Sir Simon Stevens, Amanda Pritchard March 2020
-
Letter: Supporting doctors in the event of a COVID19 epidemic in the UK
Letter from the CMOs for Wales, Scotland, Northern Ireland and England, and the National Medical Director of NHS England and NHS Improvement and the Medical Director of the GMC.
College conferences
Resources: College conferences
-
Conferences
The College aims to make a direct contribution to the further education of pathologists and medical professionals.